How much ammonia is too much? The quick answer is: if a test kit is able to measure it, you've got too much (i.e., it's in a high enough concentrations to stress fish). Consider emergency action (water changes and zeolite clay) to reduce the danger. (A more detailed discussion of ammonia toxicity can be found later in this section.)
In aquaria-speak, the ``nitrogen cycle'' (more precisely, the nitrification cycle) is the biological process that converts ammonia into other, relatively harmless nitrogen compounds. Fortunately, several species of bacteria do this conversion for us. Some species convert ammonia (NH3) to nitrite (N02-), while others convert nitrite to nitrate (NO3-). Thus, cycling the tank refers to the process of establishing bacterial colonies in the filter bed that convert ammonia -> nitrite -> nitrate.
The desired species of nitrifying bacteria are present everywhere (e.g., in the air). Therefore, once you have an ammonia source in your tank, it's only a matter of time before the desired bacteria establish a colony in your filter bed. The most common way to do this is to place one or two (emphasis on one or two) hardy and inexpensive fish in your aquarium. The fish waste contains the ammonia on which the bacteria live. Don't overfeed them! More food means more ammonia! Some suggested species include: common goldfish (for cold water tanks), zebra danios and barbs for warmer tanks, and damselfishes in marine systems. Note: Do not use ``toughies'' or other feeder fishes. Although cheap, they are extremely unhealthy and using them may introduce unwanted diseases to your tank.
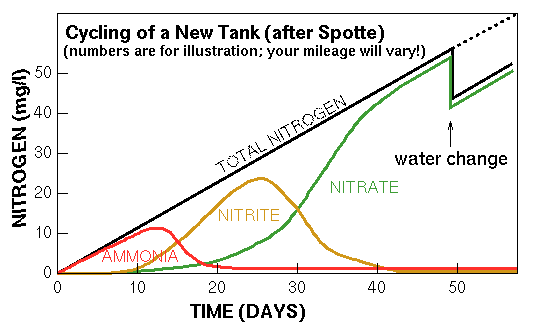
During the cycling process, ammonia levels will go up and then suddenly plummet as the nitrite-forming bacteria take hold. Because nitrate-forming bacteria don't even begin to appear until nitrite is present in significant quantities, nitrite levels skyrocket (as the built-up ammonia is converted), continuing to rise as the continually-produced ammonia is converted to nitrite. Once the nitrate-forming bacteria take hold, nitrite levels fall, nitrate levels rise, and the tank is fully cycled.
Your tank is fully cycled once nitrates are being produced (and ammonia and nitrite levels are zero). To determine when the cycle has completed, buy appropriate test kits (see the TEST KIT section) and measure the levels yourself, or bring water samples to your fish store and let them perform the test for you (perhaps for a small fee). The cycling process normally takes anywhere from 2-6 weeks. At temperatures below 70F, it takes even longer to cycle a tank. In comparison to other types of bacteria, nitrifying bacteria grow slowly. Under optimal conditions, it takes fully 15 hours for a colony to double in size!
It is sometimes possible to speed up the cycling time. Some common procedures for this are detailed later in this section.
Warning: AVOID THE TEMPTATION TO GET MORE FISH UNTIL AFTER YOUR TANK HAS FULLY CYCLED! More fish means more ammonia production, increasing the stress on all fish and the likelihood of fish deaths. Once ammonia levels reach highly stressful or toxic levels, your tank has succumbed to ``New Tank Syndrome''; the tank has not yet fully cycled, and the accumulating ammonia has concentrations lethal to your fish.
The exact concentration at which ammonia becomes toxic to fish varies among species; some are more tolerant than others. In addition, other factors like water temperature and chemistry play a significant role. For example, ammonia (NH3) continually changes to ammonium (NH4+) and vice versa, with the relative concentrations of each depending on the water's temperature and pH. Ammonia is extremely toxic; ammonium is relatively harmless. At higher temperatures and pH, more of the nitrogen is in the toxic ammonia form than at lower pH.
Standard test kits measure total ammonia (ammonia plus ammonium) without distinguishing between the two forms. The following chart gives the maximum long-term level of ammonia-N in mg/L (ppm) that can be considered safe at a given temperature and pH. Again, note that a tank with an established biological filter will have no detectable ammonia; this chart is provided only for emergency purposes. If your levels approach or exceed the levels shown, take emergency action IMMEDIATELY.
Water Temperature pH 20C (68F) 25C (77F) _________________________________ 6.5 15.4 11.1 7.0 5.0 3.6 7.5 1.6 1.2 8.0 0.5 0.4 8.5 0.2 0.1
As a final caution, several commercial products (e.g., ``Amquel'' or ``Ammo-Lock'') safely neutralize ammonia's toxicity. Amquel does not remove the ammonia, it simply neutralizes its toxicity. Biological filtration is still needed to convert the (neutralized) ammonia to nitrite and nitrate. Thus, adding Amquel causes the ammonia produced by the fish to be neutralized instantly, yet still allows the nitrogen cycle to proceed. Using Amquel during the cycling phase has one significant drawback, however. Amquel (and similar products) may cause ammonia test kits to give false readings, making it difficult to determine exactly when cycling has completed. See the TEST KIT SECTION for details.
It is also possible to cycle a tank without ever adding fish. The role fish provide in the cycling process is simply their steady production of ammonia; the same effect can be achieved by adding chemical forms of ammonia manually (e.g., ammonium chloride). However, it is a bit more complicated than using fish because the water chemistry needs to be monitored more closely in order to add the proper amount of ammonia on a day-to-day basis.
Most filters have some sort of foam block or floss insert on which nitrifying bacteria attach. Borrowing all or part of such an insert and placing it in the new tank's filter gets things going more quickly.
If the established tank uses an undergravel filter, nitrifying bacteria will be attached to the gravel. Take some of the gravel (a cup or more) and hang it in a mesh bag in your filter (if you can), or lay it over the top of the gravel in the new tank (if it has an UGF).
If you have a box, sponge or corner filter, simply connect it to an established aquarium and let it run for a week or so. Bacteria in the water will establish a bed in the new filter. After a week, move the now ``seasoned'' filter to the new tank.
More recently, products containing colonies of nitrifying bacteria have become available at pet shops (e.g., ``Fritz'', ``Bio-zyme'', ``Cycle''). In theory, adding the bacteria jump-starts the colonization process as above. Net experience with such products has been mixed; some folks report success, while others report they don't work at all. In principle, such products should work well. However, nitrifying bacteria cannot live indefinitely without oxygen and food. Thus, the effectiveness of a product depends on its freshness and can be adversely effected by poor handling (e.g., overheating). Unfortunately, these products don't come with a freshness date, so there is no way to know how old they are.
Some (not many) aquarium stores will provide aquarium buyers with a cup of gravel from an established tank. A word of caution is appropriate here. Due to the nature of the business, tanks in stores are very likely to contain unwanted pathogens (bacteria, parasites, etc.); you don't want to add them to an established tank. For someone setting up their very first tank, however, all fish will probably be purchased from the same store, so the danger is relatively small, as the newly purchased fish will have been exposed to the same pathogens. If possible, seed a filter with bacteria from a non-store tank.
Of course, there are many variations on the above that work. However, it is a bit difficult to give an exact recipe that is guaranteed to work. It is advisable to take a conservative approach and not add fish too quickly. In addition, testing the water to be sure nitrates are being produced eliminates the guesswork of determining when your tank has cycled.